Stay Informed


Fighting cancer starts by finding its fingerprint
The tumor fingerprint
Oncology is evolving from thinking about cancer according to site of origin to thinking about cancer according to tumor genomics1-6
Personalized cancer therapy
The diagnosis and management of cancer is being revolutionized by precision oncology, which defines cancers by their underlying genomic changes.1,2,7,8
A laboratory method used to make many copies of a specific genetic sequence for analysis. The DNA copies help tell whether a specific mRNA molecule is being made and may be used to look for certain changes in a gene or chromosome or for activation of certain genes.
Source: National Cancer Institute. Accessed February 17, 2023. https://www.cancer.gov/publications/dictionaries/cancer-terms/def/RT-PCR
A method used to look at genes or chromosomes in cells and tissues. FISH can be used to identify where a gene is located, how many copies of the gene are present, and if there are any chromosomal abnormalities.
Source: National Cancer Institute. Accessed February 17, 2023. https://www.cancer.gov/publications/dictionaries/cancer-terms/def/fish
A laboratory method that uses antibodies to check for certain antigens in a sample of tissue. IHC is used to help diagnose cancer and help tell the difference between different types of cancer.
Source: National Cancer Institute. Accessed February 17, 2023. https://www.cancer.gov/publications/dictionaries/cancer-terms/def/immunohistochemistry
The goal of precision oncology is to optimize and tailor each patient's treatment approach based on the genomic profile of the patient's cancer.6
Advancements in precision oncology
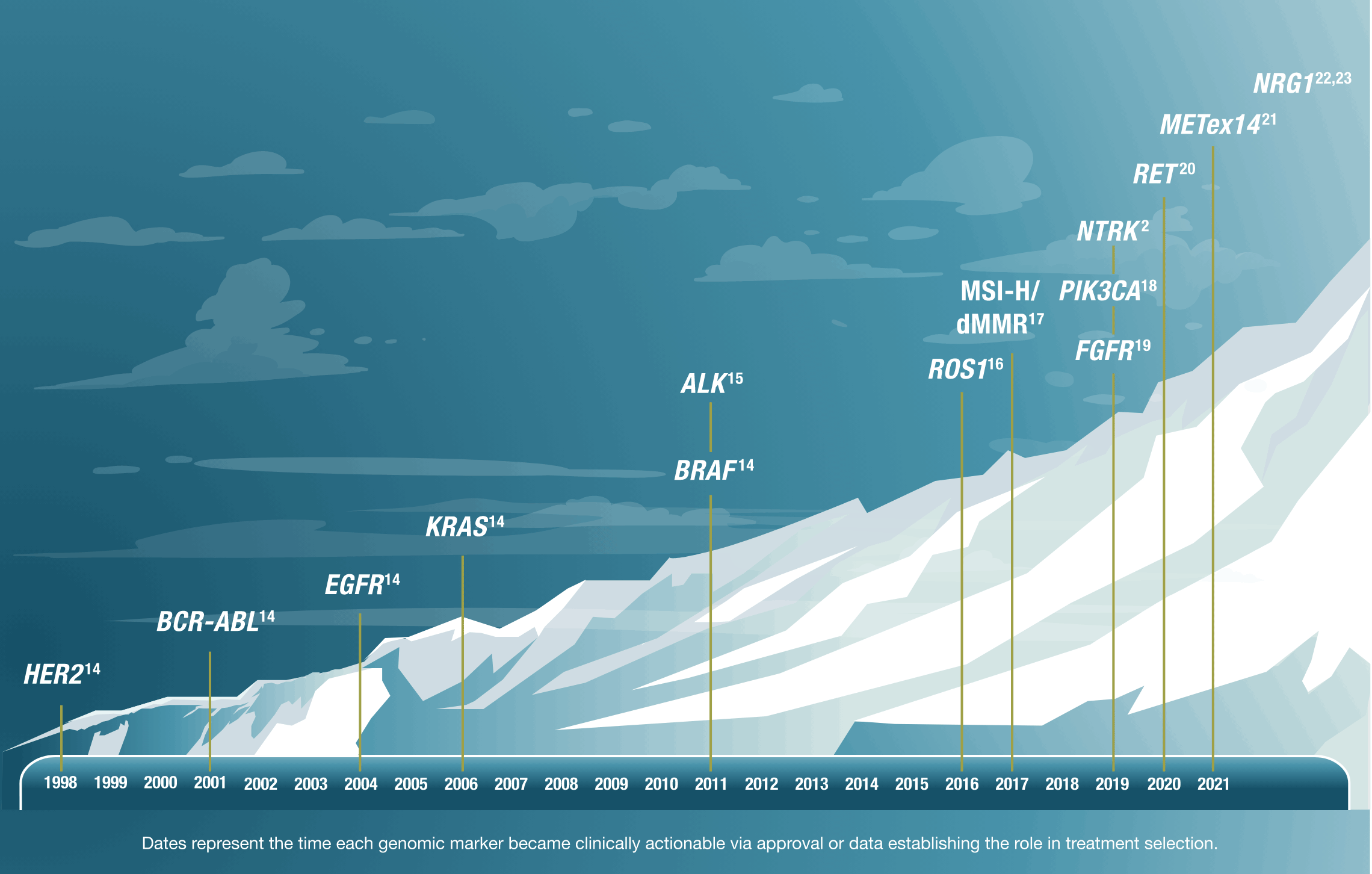
How are genomics changing the future of oncology?
Understanding the genomic profile and oncogenic drivers of a patient's cancer can help determine a more tailored approach to care.9,10
Individual genomic alterations may be rare; however, alterations in totality are found in a significant percentage of patients with cancer11-13,a

It is estimated that
>50%
of patients may have
a potential
a potential
actionable
alteration
11-13,aalteration
A genomic finding that provides justification for medical intervention and for which a targeted therapy is available.
Source: Yates LR et al. Ann Oncol. 2018;29(1):30-35. doi:10.1093/annonc/mdx707
aLarge retrospective series have documented that 80% to 90% of patients tested will have potentially actionable genomic alterations, although the definition of actionable can vary substantially.11
As our understanding of cancer biology advances, both the number and rate of discovery of actionable genomic alterations continue to rise2
Targeting genomic alterations can potentially lead to better outcomes for patients
See the data

FISH, fluorescence in situ hybridization; IHC, immunohistochemistry; MSI-H/dMMR, microsatellite instability-high/mismatch repair deficiency; NGS, next-generation sequencing; RT-PCR, reverse transcription-polymerase chain reaction.
References: 1. Adashek JJ, Subbiah V, Kurzrock R. From tissue-agnostic to N-of-one therapies: (r)evolution of the precision paradigm. Trends Cancer. 2021;7(1):15-28. doi:10.1016/j.trecan.2020.08.009 2. Malone ER, Oliva M, Sabatini PJB, Stockley TL, Siu LL. Molecular profiling for precision cancer therapies. Genome Med. 2020;12(1):8. doi:10.1186/s13073-019-0703-1 3. Doroshow DB, Doroshow JH. Genomics and the history of precision oncology. Surg Oncol Clin N Am. 2020;29(1):35-49. doi:10.1016/j.soc.2019.08.003 4. Tsimberidou AM, Fountzilas E, Nikanjam M, Kurzrock R. Review of precision cancer medicine: evolution of the treatment paradigm. Cancer Treat Rev. 2020;86:102019. doi:10.1016/j.ctrv.2020.102019 5. Lassen UN, Makaroff LE, Stenzinger A, et al. Precision oncology: a clinical and patient perspective. Future Oncol. 2021;17(30):3995-4009. doi:10.2217/fon-2021-0688 6. Rodriguez-Rodriguez L, Hirshfield KM, Ganesan S. Preface: Introduction to precision medicine oncology. In: Rodriguez-Rodriguez L, ed. Precision Medicine Oncology: A Primer. Rutgers University Press; 2020:ix-xiii. 7. Personalized Medicine Coalition. The Personalized Medicine Report 2020: Opportunities, Challenges, and the Future. Accessed February 7, 2023. https://www.personalizedmedicinecoalition.org/Userfiles/PMC-Corporate/file/PMC_The_Personalized_Medicine_Report_Opportunity_Challenges_and_the_Future.pdf 8. Shin DH, Lee D, Hong DW, et al. Oncogenic function and clinical implications of SLC3A2-NRG1 fusion in invasive mucinous adenocarcinoma of the lung. Oncotarget. 2016;7(43):69450-69465. doi:10.18632/oncotarget.11913 9. El-Deiry WS, Goldberg RM, Lenz H-J, et al. The current state of molecular testing in the treatment of patients with solid tumors, 2019. CA Cancer J Clin. 2019;69(4):305-343. doi:10.3322/caac.21560 10. Faulkner E, Holtorf A-P, Walton S, et al. Being precise about precision medicine: What should value frameworks incorporate to address precision medicine? A report of the Personalized Precision Medicine Special Interest Group. Value Health. 2020;23(5):529-539. doi:10.1016/j.jval.2019.11.010 11. Schwartzberg L, Kim ES, Liu D, Schrag D. Precision oncology: who, how, what, when, and when not? Am Soc Clin Oncol Educ Book. 2017;37:160-169. doi:10.1200/EDBK_174176 12. Kris MG, Johnson BE, Berry LD, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA. 2014;311(19):1998-2006. doi:10.1001/jama.2014.3741 13. Priestley P, Baber J, Lolkema MP, et al. Pan-cancer whole-genome analyses of metastatic solid tumours. Nature. 2019;575(7781):210-216. doi:10.1038/s41586-019-1689-y 14. Colomer R, Mondejar R, Romero-Laorden N, Alfranca A, Sanchez-Madrid F, Quintela-Fandino M. When should we order a next generation sequencing test in a patient with cancer? EClinicalMedicine. 2020;25:100487. doi:10.1016/j.eclinm.2020.100487 15. Kazandjian D, Blumenthal GM, Chen H-Y, et al. FDA approval summary: crizotinib for the treatment of metastatic non-small cell lung cancer with anaplastic lymphoma kinase rearrangements. Oncologist. 2014;19(10):e5-e11. doi:10.1634/theoncologist.2014-0241 16. Kazandjian D, Blumenthal GM, Luo L, et al. Benefit-risk summary of crizotinib for the treatment of patients with ROS1 alteration-positive, metastatic non-small cell lung cancer. Oncologist. 2016;21(8):974-980. doi:10.1634/theoncologist.2016-0101 17. Marcus L, Lemery SJ, Keegan P, Pazdur R. FDA approval summary: pembrolizumab for the treatment of microsatellite instability-high solid tumors. Clin Cancer Res. 2019;25(13):3753-3758. doi:10.1158/1078-0432.CCR-18-4070 18. Narayan P, Prowell TM, Gao JJ, et al. FDA approval summary: alpelisib plus fulvestrant for patients with HR-positive, HER2-negative, PIK3CA-mutated, advanced or metastatic breast cancer. Clin Cancer Res. 2021;27(7):1842-1849. doi:10.1158/1078-0432.CCR-20-3652 19. Sayegh N, Tripathi N, Agarwal N, Swami U. Clinical evidence and selecting patients for treatment with erdafitinib in advanced urothelial carcinoma. Onco Targets Ther. 2022;15:1047-1055. doi:10.2147/OTT.S318332 20. Bradford D, Larkins E, Mushti SL, et al. FDA approval summary: selpercatinib for the treatment of lung and thyroid cancers with RET gene mutations or fusions. Clin Cancer Res. 2021;27(8):2130-2135. doi:10.1158/1078-0432.CCR-20-3558 21. Desai A, Cuellar S. The current landscape for METex14 skipping mutations in non–small cell lung cancer. J Adv Pract Oncol. 2022;13(5):539-544. doi:10.6004/jadpro.2022.13.5.8 22. Schram AM, Odintsov I, Espinosa-Cotton M, et al. Zenocutuzumab, a HER2xHER3 bispecific antibody, is effective therapy for tumors driven by NRG1 gene rearrangements. Cancer Discov. 2022;12(5):1233-1247. doi:10.1158/2159-8290.CD-21-1119 23. Geuijen CAW, De Nardis C, Maussang D, et al. Unbiased combinatorial screening identifies a bispecific IgG1 that potently inhibits HER3 signaling via HER2-guided ligand blockade. Cancer Cell. 2018;33(5):922-936.e10. doi:10.1016/j.ccell.2018.04.003